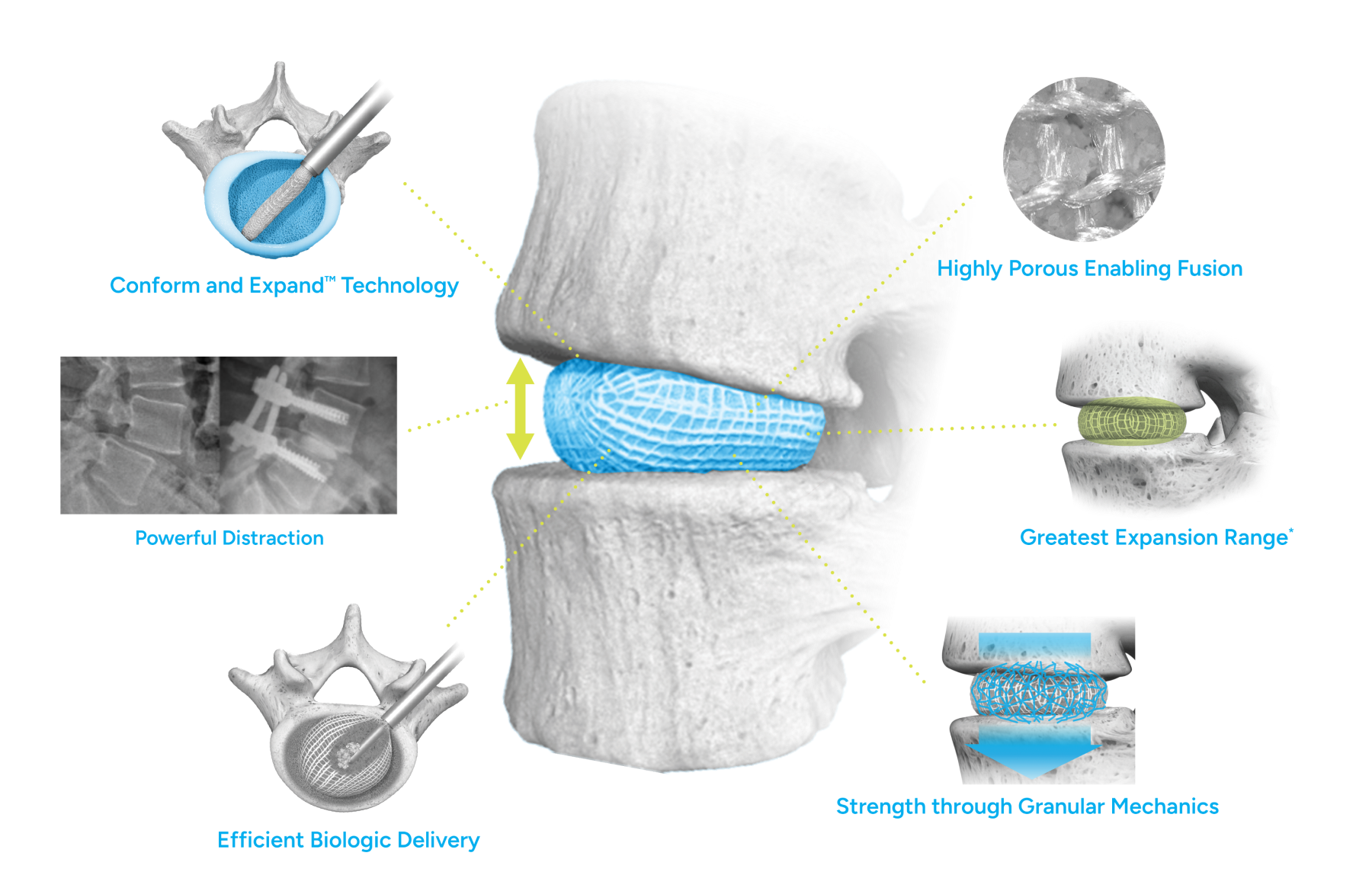
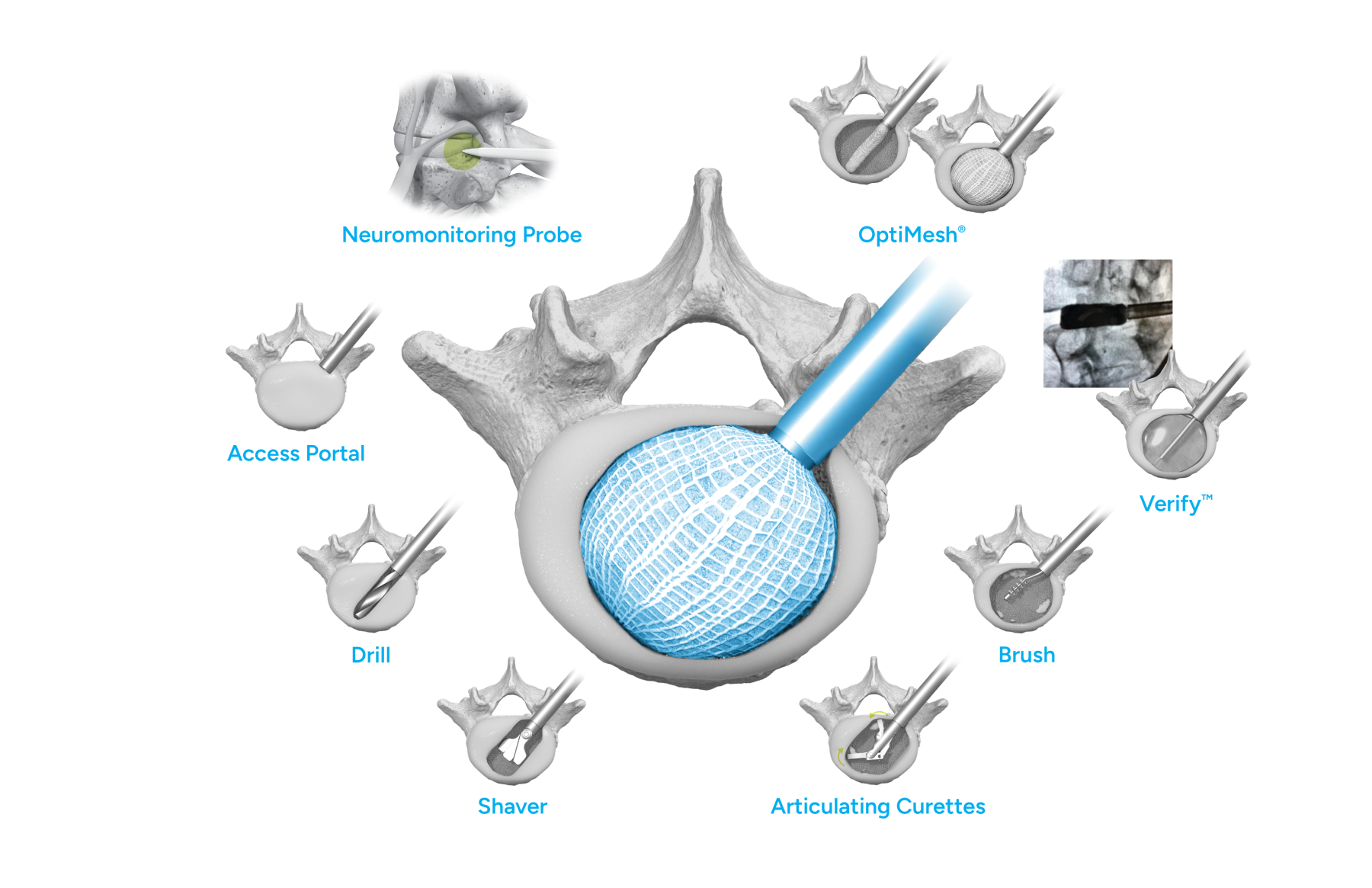
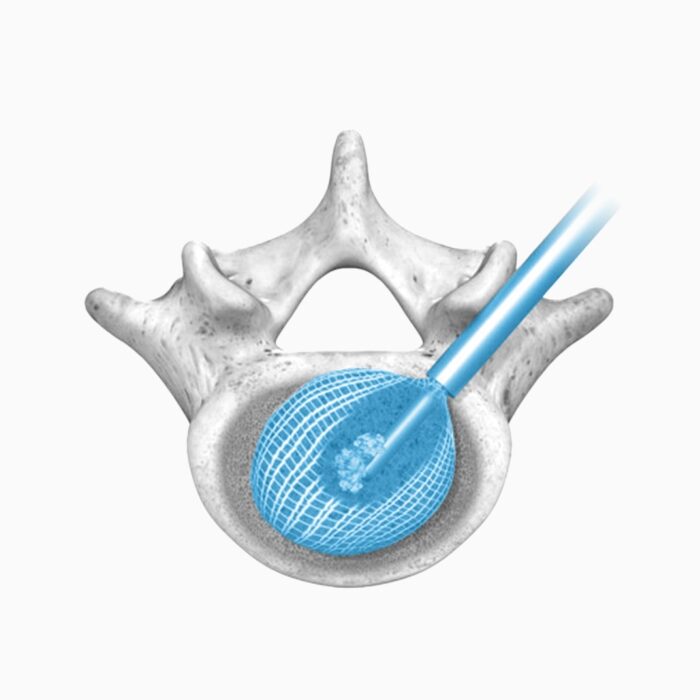
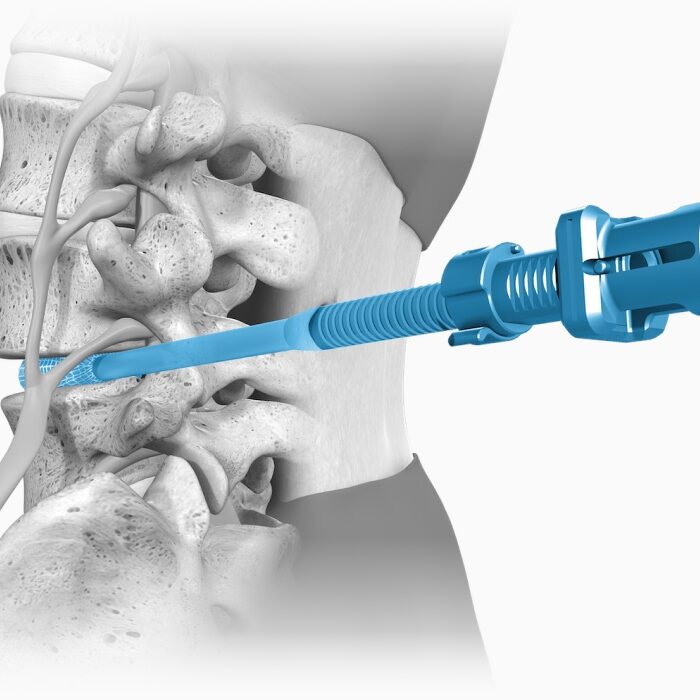
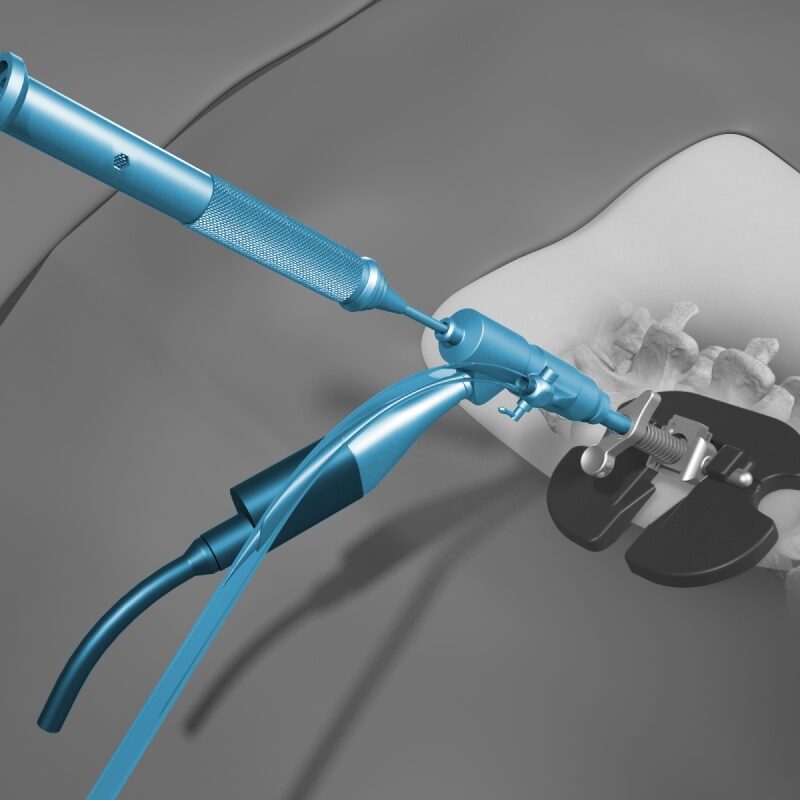
Conform and Expand™ Technology
Conform and Expand technology is designed to provide a custom anatomical fit for lumbar fusion surgical patients. OptiMesh’s powerful distraction forces and conformance to the endplates provide strength and stability to restore disc height, achieve alignment goals, and promote a robust fusion.1
Unmatched Clinical Experience
The SCOUT IDE Study
Unique in design and in published data, OptiMesh holds the highest achievement in clinical validation with an IDE De Novo level grant. Spineology Clinical Outcomes Trial (SCOUT Study): IDE trial of a novel, conformable device placed in the disc space through a small portal and filled with bone graft in-situ. At 24 months:
0%
98% fusion at 12 months post-op, evaluated by two independent radiologists.0%
Patients experienced on average, a 67% reduction in back pain at 3 months.0%
51% of patients on average experienced an improvement in back function by month 3 post-op.0%
92% of patients rated their satisfaction "excellent" or "good" at 6 months.Chi J, Nunley P, et al. Two-Year Outcomes From a Prospective Multicenter Investigation Device Trial of a Novel Conformal Mesh Interbody Fusion Device. Int J Spine Surg. 2021 Dec; 15(6): 1103-1114.

Unique in Data
20 Clinical Publications
> 50,000 implants
Additionally, 20+ years of clinical experience, 20 clinical publications, and > 50,000 devices implanted set OptiMesh alone in the interbody fusion space.